All manuscripts submitted to the ASHA Journals should adhere to the following general formatting guidelines:
Manuscripts submitted to the ASHA journals should include line numbers. Adding line numbers in Microsoft Word and many other major word processors is straightforward.
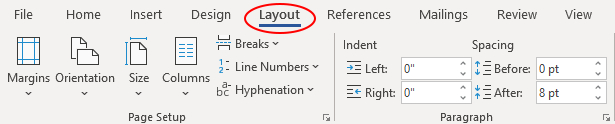
To add line numbering in Microsoft Word, start by opening the document for your manuscript and then select the layout tab in the upper toolbar (circled below).

Then, select the “Line Numbers” option in the Page Setup panel (circled below).
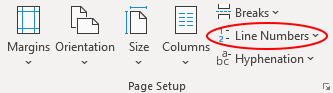
Finally, select the “Continuous” option from the Line Numbers drop-down menu (circled below) and the numbers will be automatically added.
Generally, scientific manuscripts should be organized as follows:
Because scientific papers are organized in this way, readers know what to expect from each part of the paper and they can quickly locate specific information.
A guideline of 40 pages (including title page, abstract, text, acknowledgments, references, appendixes, tables, and figures) is suggested as an upper limit for manuscript length for most manuscript types. This page limit does not include supplemental materials. Please note that this is just a general guideline. Longer manuscripts, particularly for critical reviews and extended data-based reports, will be considered, but the author(s) should submit a cover letter providing a rationale for why the added length is needed. There is not a prescribed limit on the number of tables or figures that can be included.
The title should be short and clear, yet provide a sufficient description of the work. As the title becomes the basis for most online searches, it should contain the key words describing the work presented. If your title is insufficient, people will have difficulty finding your article. The title page should also include a list of the authors and their affiliations (see Authorship Criteria and Guidelines for more information).
The abstract helps readers scan through lists of articles or search results and is essential for helping users decide whether to read the rest of the article or save it for future reference. As a result, abstracts must be brief but also informative enough to be genuinely useful.
ASHA recommends that abstracts be 150–250 words. The size limit for what can be included in your submission is set above 300 words, but that is so that very detailed abstracts for specific types of studies can be accommodated (see, for example, the abstract for this randomized controlled trial reported according to the CONSORT framework)
Regardless of the type of manuscript, abstracts must be structured using the following sections:
Purpose: The Purpose section must include a concise statement of the specific purposes, questions addressed, and/or hypotheses tested. Lengthy descriptions of rationale are not necessary or desirable.
Method: The Method section must describe characteristics and numbers of participants and provide information related to the design of the study (e.g., pre–post group study of treatment outcomes, randomized controlled trial, multiple baseline across behaviors; ethnographic study with qualitative analysis; prospective longitudinal study) and data collection methods. If the participants have been assigned randomly to study conditions, this must be noted explicitly, regardless of the design used. If the article is not data-based, information should be provided on the methods used to collect information (e.g., computerized database search), to summarize previously reported data and to organize the presentation and arguments (e.g., meta-analysis, narrative review).
Results: The Results section should summarize findings as they apply directly to the stated purposes of the article. Statistical outcomes may be summarized, but no statistics other than effect sizes should be provided within the abstract. This section may be omitted from articles that are not data-based.
Conclusions: The Conclusions section must state specifically the extent to which the stated purposes of the article have been met. Comments on the generalizability of the results (i.e., external validity), needs for further research, and clinical implications often are highly desirable.
The introduction usually describes the theoretical background, indicates why the work is important, states a specific research question, and poses a specific hypothesis to be tested. This section should provide your statement of purpose and rationale.
The methods section must provide a clear and precise explanation of how you carried out the study and why specific experimental procedures were chosen. This section describes both the techniques and the overall experimental strategy used by the authors in order to address any questions the readers may have about the experimental design. The methods section must be written with enough information so that (1) the experiment could be repeated by others to determine if the results can be replicated and (2) the audience can judge the study’s validity.
The results section contains the data collected during your study and is the heart of a scientific paper. The body of the results section is a text-based presentation of the key findings which includes references to each of the Tables and Figures. Much of the important information may be in the form of tables or graphs. The text should guide the reader through the results stressing the key results that provide the answers to the question(s) investigated.
The discussion section should explain what the results mean and how the results relate to other studies. This section interprets your findings, evaluates the hypotheses or research questions, discusses unexpected results, and ties the findings to the previous literature (discussed first in the Introduction). Any possible objections to the work and/or suggestions of areas for improvement in future research can be addressed in this section.
Citation of grant or contract support of research must be given in an acknowledgments section at the end of the article (before the References). If any part of the research was supported by an institution not named on the title page, that institution should be acknowledged in this section. Individuals who assisted in the research may be acknowledged. Do not name individuals (editors and reviewers) who participated in the review process.
Effective January 1, 2022, all ASHA journals require authors to provide a data availability statement (DAS), detailing where data supporting the results reported in the article can be found, including, where applicable, hyperlinks to publicly archived datasets analyzed or generated during the study.
To learn more about ASHA journals’ research data standards and the data availability statement, visit the Research Data Standards page.
All literature cited in the text, as well as test and assessment tools, ANSI and ISO standards, and specialized software, must be listed in this section. References should be listed alphabetically, then chronologically under each author. Journal names should be spelled out and italicized. Pay particular attention to accuracy and APA style for references cited in the text and listed in the references.
Tables present lists of numbers or text in columns, each column having a title or label. Figures are visual presentations of results, including graphs, diagrams, photos, drawings, schematics, maps, etc. Each table or figure should appear on its own page (i.e., don’t put more than one figure or table on the same page). Use arabic numerals to identify both tables and figures, and do not use suffix letters for complex tables. Instead, simplify complex tables by making two or more separate tables. Table titles and figure captions should be concise but explanatory. The reader should not have to refer to the text to decipher the information. Keep in mind the width of a column or page when designing tables and figures. In other words, consider whether legibility will be lost when reductions are made to fit a column or page width. Avoid “special effects” in figures (e.g., three-dimensional bar graphs) because they distort, rather than enhance, the data and distract the reader.
Perspectives of the ASHA Special Interest Groups authors should—and other journal authors are encouraged to—include up to 3 learning outcomes in order to help create learning assessments for ASHA Continuing Education Units (CEUs) . ASHA Continuing Education provides guidelines to crafting learning outcomes .
An appendix is an optional part of the paper that allows you to include detailed information that would interrupt the flow of the main body of the article. Examples of items you might have in an appendix include lists of words, a questionnaire or tool used in the study, a detailed description of an apparatus used in the research, etc.
Supplemental material is nonessential to understanding of the paper, but may present information that further enhances the article. Examples of the types of material and file formats accepted may be found on the Supplemental Material and Multimedia section of this page. Any files for supplemental materials should be submitted at the same time as the manuscript and will be subject to the normal peer review process. Please indicate clearly that the material is intended as supplementary, and be sure that it is referred to within the text of the manuscript. Also, please provide a concise (1- or 2-sentence) description for each file supplied.
The ASHA Journals use the Publication Manual of the American Psychological Association (7th ed.) for reference style and formatting. You can purchase your own copy of the APA Publication Manual directly through their website, or various other retailers. Please use the quick resources box to the right for various other online materials which may prove helpful when formatting your references.
ASHA has partnered with Figshare to enable authors to automatically archive data and supporting materials in an open access, public repository when submitting an article to an ASHA journal. Figshare provides unlimited data storage for a variety of file formats. All content is assigned a permanent web link (DOI) so you and other authors can link directly to it from future papers. You can easily upload supplemental files within the existing Editorial Manager submission workflow. This supplemental material can consist of any of the following:
If you plan to take advantage of this service, then your data and other supplemental material must be submitted before your article is accepted. If your article is accepted for publication, then all of your supplemental files are automatically deposited into the ASHA Journals Figshare data repository without charge. Figshare is an open access repository using Creative Commons licenses for supplemental material hosted there. CC BY is the license used for most file types. CC0 is the standard license used for sharing data and databases. However, you can select another license to set access restrictions on your supplemental material if needed. Please review the explanation of Creative Commons licenses for more information.
Authors who have video or animation files that they wish to submit with their article are encouraged to include links to these within the body of the article. This can be done in the same way as a figure or table by referring to the video or animation content and noting in the body text where it should be placed. All submitted files should be properly labeled so that they directly relate to the file’s content. In order to ensure that your video, audio, or animation material is directly usable, please provide the files in one of our recommended file formats (see below). Video, audio, and animation files supplied will be published online in the electronic version of your article.
Please supply “stills” with your files: you can choose any frame from the video or animation or create a separate image. For audio clips, you can select a thumbnail image that you feel is representative of the content of the audio clip. These will be used instead of standard icons and will personalize the link to your multimedia data. Because video, audio, and animation cannot be embedded in the print version of the journal, please provide text for both the electronic and the print version for the portions of the article that refer to this content.
If the content being submitted is truly “supplementary” (not essential to the content of the article or only of supplementary interest to the reader), it can be included as Supplementary Content (i.e., accessible only electronically via an active link in the article). Multimedia files included as Supplementary Content should be referred to at an appropriate place in the text. If this is not done, any Supplementary Content will be referred to in an appendix without specifying exactly what it is. All submitted files should be properly labelled so that they directly relate to the file’s content. This will ensure that the files are fully searchable by users. If the author does not hold copyright to the video, the author must obtain permission for the video to be published in the journal. This permission must be for unrestricted use in all print, online, and licensed versions of the journal. Please see our guide on seeking permission for more information.
Figshare allows the upload of any file type and is able to visualize hundreds of different file types in the browser window. Please visit the Figshare Knowledge site for more information on file types for Figshare.
If you are not sure what specifications to use to create a video, the following settings are recommended:
Image Recommendations
Any files for supplemental materials should be submitted at the same time as the manuscript and will be subject to the normal peer review process. Please indicate clearly that the material is intended as supplementary, and be sure that it is referred to within the text of the manuscript. Also, please provide a concise (1- or 2-sentence) description for each file supplied. The material must be original content that has not been previously published. Where possible, the material will be copyedited. Please note: Recordings or images that involve identifiable participants require permission from those individuals. Please secure and provide that signed consent.
When publishing identifiable images, or video and audio recordings, from human research participants in ASHA journals, authors include a statement in the published paper affirming that they have obtained informed consent for publication of the images and/or recordings.
The consent form should cover both image and voice of the person (if both are used); should specifically grant consent for submitting the recording for publication in a scientific journal; and should include online publishing if the recording and/or still images from it for purposes related to promotion of the published study.
All reasonable measures must be taken to protect patient anonymity. Black bars over the eyes are not acceptable means of anonymization. In certain cases, ASHA may insist upon obtaining evidence of informed consent from authors. Images and recordings without appropriate consent will be removed from publication.